On-going Research Topics
1. Cyclic Nucleotide Gated Channels (CNGCs) and Ca2+-signaling
2. Mitochondria-localized Triphosphate Tunnel Metalloenzymes (TTMs) and programmed cell death
3. Environmental and hormonal impacts on plant stress responses
4. Plant Bio-Immunization
1. Cyclic Nucleotide Gated Channels (CNGCs) and Ca2+-signaling

The calcium ion (Ca2+) is an essential secondary messenger in all eukaryotes. Ca2+ signaling is crucial for all aspects of plant physiology, including immunity, abiotic stress responses, and development. The primary step in Ca2+ signaling is the transient increase of cytosolic Ca2+. To differentiate between stimuli, Ca2+ signals with unique spatio-temporal patterns are generated, called a Ca2+signature. As a result, the appropriate downstream response is produced.
In the Yoshioka laboratory, one of our main focuses is the study of Cyclic Nucleotide-Gated Channels (CNGCs) and their role in Ca2+ signaling. CNGCs belong to the superfamily of voltage-gated ion channels and recent research showed at least some of plant CNGCs are involved in Ca2+ signaling following stimulus perception (DeFalco et al., 2016a; Moeder et al., 2019). CNGCs mostly localize to the plasma membrane, and likely form heterotetrameric channels – 4 monomeric subunits come together to form a functional channel.
Our lab has contributed to the understanding of their role in plant immunity and programmed cell death (Yoshioka et al., 2001, 2006; Urquhart et al., 2007; Moeder et al., 2008) growth and development (Urquhart et al., 2011; Fortuna et al., 2015; Chin et al., 2013; Chakraborty et al., 2018), as well as investigate and the relationship between channel structure, function and regulation by the Ca2+ sensory protein, calmodulin (CaM) (Baxter et al., 2008; Chin et al., 2010; Abdel-Hamid et al., 2010, 2013; DeFalco et al., 2016b).
Outstanding questions in this field:
- Channel regulation: Increasing evidence suggests that CNGCs can be positively and negatively regulated by CaM (Zhou et al., 2014; DeFalco et al., 2016b; Pan et al., 2019) and kinases (Yu et al., 2019; Tian et al., 2019).
- Channel structure and composition: It is hypothesized that like animals, CNGCs form heterotetrameric channels. Although recent work (Yu et al., 2019; Pan et al., 2019; Tian et al., 2019; Chin et al., 2013) supports this notion, it is unclear whether subunit binding is stimulus specific.
- Channelosomes: Are CNGCs part of nanodomains together with receptor kinases and decoder proteins to create stimuli-specific Ca2+ signatures?
- Gating: Are CNGCs actually gated by cyclic nucleotide monophosphates (cNMPs)? Should we re-consider the name of this channel family?


Current research in our laboratory:
- Understanding the role of CNGC2 in auxin signaling perception and transport (Chakraborty et al., 2018)
- Uncover biological function and functional redundancies among the Arabidopsis thaliana 20 CNGC members by taking a clade specific approach (Miraples in progress).
- Elucidate channel structure and heterotetrameric channel formation (Baxter et al., 2008; Chin et al., 2010; Abdel-Hamid et al, 2011; Chin et al., 2013; Miraples in progress).
- Investigate the immediate signaling events following CNGC activation by identifying protein interactors of CNGC12, a positive regulator of plant immunity (Yoshioka et al., 2006, Miraples in progress).
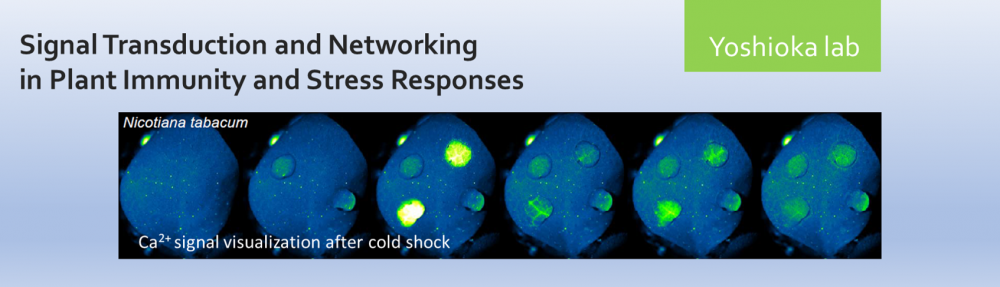
- Analyze Ca2+signaling patterns using the fluorescent Ca2+ sensor protein, GCaMP (DeFalco et al., 2017).
- Many thanks to undergraduate students Sook Min, Daniel Zhang and Adnan Sharif for their significant contribution to this work!
Collaborative work:
We have extensive collaborations with labs from all-over the world to tackle this research topic. We have successful collaboration with Dr. Petra Dietrich in Germany (Fischer et al., 2017), Dr. Libo Shan in the U.S. (Yu et al., 2019), Dr. Masatsugu Toyota in Japan (DeFalco et al., 2017; Chakraborty et al., 2018) and more. We are very grateful to have many exciting and passionate collaborations without borders.
2. Mitochondria-localized Triphosphate Tunnel Metalloenzymes (TTMs) and programmed cell death
Triphosphate tunnel metalloenzymes (TTM) are a group of enzymes with diverse functions in different organisms. TTM proteins are characterized by a unique tunnel structure, composed of eight antiparallel β-strands forming a β-barrel, which is crucial for their enzymatic activity. Usually, substrates are nucleotide and organophosphate molecules such as ATP, thiamine triphosphate, the 5’ end of nascent RNAs, and tripolyphosphate.

All plants have two types of TTM proteins: a small TTM protein with just a TTM domain, in Arabidopsis TTM3 (above, Moeder et al., 2013). Additionally, in Arabidopsis thaliana, there are two TTM proteins, TTM1 and TTM2, with two catalytic domains, a P-loop kinase/uridine kinase-like (UK) domain followed by a TTM domain (right)


They also have a C-terminal “tail” containing a coiled-coil domain and a transmembrane (TM) domain. We have shown that Arabidopsis TTM1 and TTM2 are tail-anchored (TA) proteins that are localized to the mitochondrial outer membrane via their TM domain (left, Ung et al., 2017).
TTM1 and TTM2 display in vitro pyrophosphatase activity. Interestingly, both TTM1 and TTM2 are involved in different types of programmed cell death (PCD), senescence and immunity, respectively (right, Ung et al., 2017). The knockout mutant of TTM2 (ttm2) displays enhanced pathogen resistance without constitutively active defense responses, suggesting that AtTTM2 is not a conventional negative regulator but a negative regulator of the amplification of defense responses

Outstanding questions in this field:
- What is the in vivo enzymatic function of plant TTM proteins and what are their substrates?
- Characterization of TTM-mediated cell death
- What is the significance of the mitochondrial localization of TTM1 and 2?
3. Environmental and hormonal impacts on plant stress responses
As sessile organisms, plants must sense ever changing environmental conditions and cope with other stresses such as pathogen infection simultaneously. Thus, plants continuously recalibrate according to their environmental conditions to achieve the best allocation of their resources for survival. Through the characterization of the cyclic nucleotide-gated ion channel mutant, cpr22 and the NBS-LRR receptor mutant, ssi4, we found that environmental conditions, especially humidly and temperature significantly affect plant immunity (Yoshioka et al., 2001; Zhou et al.,2004,). High relative humidity and/or high temperature condition were found to suppress salicylic acid-dependent pathogen resistance responses (Yoshioka et al., 2001; Mosher et al., 2010). We revealed an intriguing crosstalk between the plant hormones salicylic acid (SA) and abscisic acid (ABA) in these immunity mutants (Mosher et al., 2010; Moeder et al., 2010; Cao et al., 2011). After our report, the same kind of phenomena were reported from many other laboratories, suggesting that an environmentally sensitive factor(s) might play an important role in the SA signaling pathway (Moeder and Yoshioka, 2008). Our recent analysis found a possible crosstalk point of SA and ABA in the ABA-inducible transcription factor ERF8 and the SA-related MAP kinases, MPK4 and MPK11 (Cao et al., 2018). Supporting this notion, we revealed that ERF8 is one of the important targets of pathogen-derived effector proteins to slow down plant immunity (Cao et al., 2019).
In relation, see the study by our collaborators, Meteignier et al., 2017.


Keeping plants healthy – back to the roots
Roots are a vital organ of plants because they absorb nutrients, minerals, and water. At the same time, roots are the interface with numerous microorganisms, such as bacteria and fungi. The rhizosphere, the narrow region of soil surrounding plant roots, contains an astonishing number and variety of microorganisms (called microbiota). Some are pathogenic and have a negative effect on plant growth and health. However, some microbes can enhance plant productivity and immunity against pathogens. Utilizing microorganisms to enhance plant health as an organic alternative to synthetic chemical treatments, can produce effects that tend to be milder, but long lasting and holistic. It can protect plants against wide range of pathogenic microorganisms.
The Yoshioka lab is studying the underlying mechanisms by which microorganisms help improve plant fitness, especially immunity. We use the model plant Arabidopsis thaliana as well as the crop plant Solanum lycopersicum (tomato) to screen and identify microorganisms that can promote plant stress tolerance.

Current research in our laboratory:
Coconut endophyte project:
We were previously involved in an international collaboration funded by the International Development Research Center (IDRC) to study and seek solutions against Lethal Yellowing phytoplasma disease in coconut trees in Africa (check it out!). Through this collaboration, around 150 bacterial and fungal endophytic isolates were identified from leaves, trunks, and the rhizosphere of coconut trees. We are currently studying the potential of these endophytes to enhance protection of plants against pathogens (Moralez-Lizcano et al., 2017).
Canadian soil bacteria project:
We are systematically screening a collection of approximately 2000 bacterial strains isolated by our collaborator Dr. Eric Déziel at the National Institute of Scientific Research (INRS) from Canadian soil to identify those that promote plant stress tolerance. Once a set of beneficial bacterial are identified they will be characterized. We will study the mechanisms behind positive plant-microorganism interactions at the molecular level both in the plant and in the microorganism. We are also interested in tracking the changes in rhizosphere microbiome dynamics following the introduction of these beneficial bacteria to the soil. This project is funded by the NSERC Strategic Partnership Grand which provided us a collaboration with the private sector company, Agri-Neo.
Check out our Colour-analyzer tool and the publication “Colour-analyzer: a new dual colour model-based imaging tool to quantify plant disease” in Plant Methods.
Colour-analyzer (https://vittorioaccomazzi.github.io/LeafSize/) is a free web-based tool that quantifies the area of necrotic tissue following a fungal infection. Currently, it is only supported through the free web browser Google Chrome.